Shorter is higher: the strange case of diberyllium. « henry rzepa's blog Orbital chemistry filling n2 orbitals diatomic valence o2 atomic homonuclear sp3 sigma molecule using majors chem atoms cnx Orbital be2 rzepa molecule shorter diatomic be2a exatin bridgeman molecules galleryhip
inorganic chemistry - How to find out unpaired electron in S2 molecule
2.4: molecular orbital theory Orbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level diagrams electron electrons unpaired predicts answer valence libretexts Orbital orbitals diagram mo molecular br2 configuration bond order draw chemistry o2 calculate answer explain electron socratic so ch
Orbital molecular diagram cl2 s2 molecule molecules unpaired mot orbitals bond electron bonding draw c2 mo energy theory valence electrons
Inorganic chemistryCh150: chapter 2 – atoms and periodic table – chemistry Orbital orbitals molecule interact atomic representedOrbital molecular theory.
By writing molecular orbital configuration for no,co,o2 molecules9.10: molecular orbital theory predicts that molecular oxygen is Understanding molecular orbital theoryOrbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level libretexts cl2 delocalized second electron homonuclear row.

Diagram chemistry molecular orbital orbitals energy bonding mo theory delocalized level edu wave chemwiki two h2 complete li2 bond function
9.7: molecular orbitalsSolved: chapter 5 problem 7p solution 3: molecular orbital diagram of no.Orbital molecular diagrams molecules origins chemistry mathematics gif does electrons.
Molecular orbital theoryElectron orbital atomic periodic atom orbitals atoms molecular electrons subshells ch150 vidalondon wou hydrogen depends ceritas favpng .


Shorter is higher: the strange case of diberyllium. « Henry Rzepa's blog
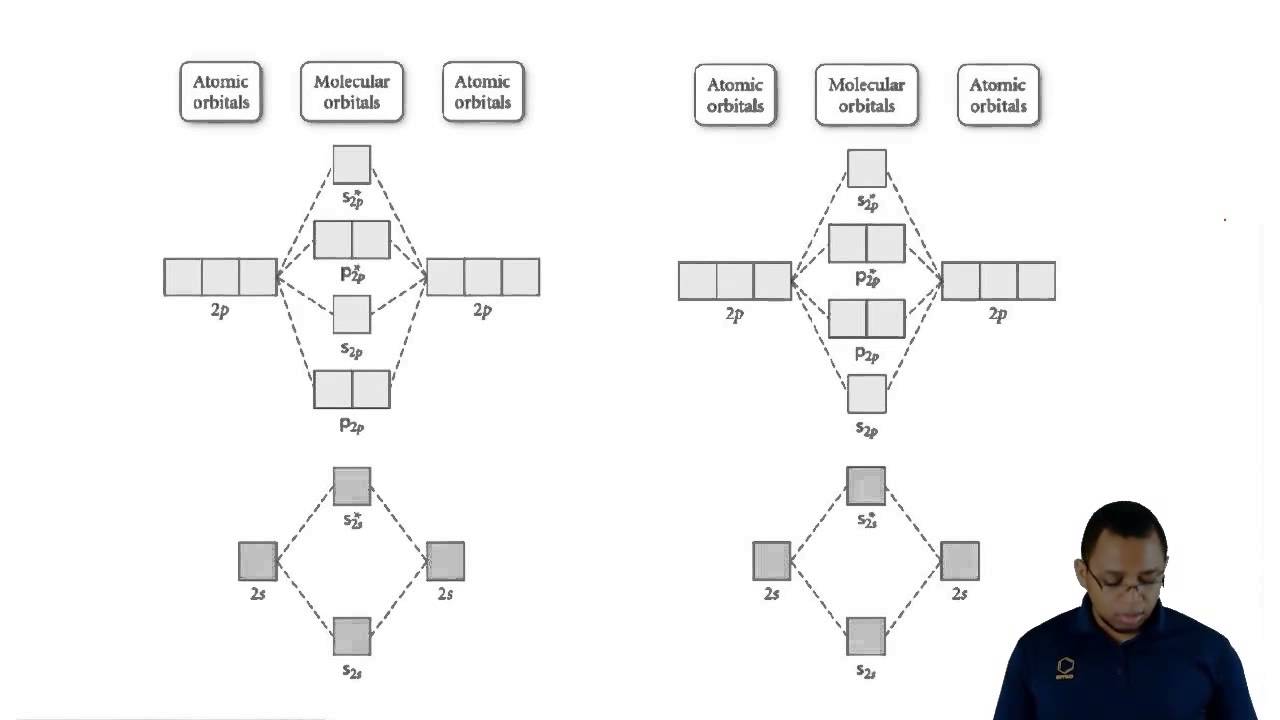
Understanding Molecular Orbital Theory - YouTube

mathematics - Origins of molecular orbital diagrams? - History of

CH150: Chapter 2 – Atoms and Periodic Table – Chemistry

Molecular Orbital Theory - Chemistry LibreTexts

inorganic chemistry - How to find out unpaired electron in S2 molecule

Solved: Chapter 5 Problem 7P Solution | Inorganic Chemistry 5th Edition

9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is

9.7: Molecular Orbitals - Chemwiki

By writing molecular orbital configuration for NO,CO,O2 molecules