Orbitals energy coordination octahedral chemistry magnetic compounds complex which axes dxz dxy five first properties dz2 axis diagram along field Ions cu2 outer ion electron atom electrons lose neither occurring Field crystal orbitals cu2 theory octahedral why than ionic zn2 spatial ligands ligand arrangement geometries chemistry other radii less libretexts
6.6: 3D Representation of Orbitals - Chemistry LibreTexts
Electronic electron ions cu2 Studies of the local distortions for cu2+ in ba2zn(hcoo)6·4h2o single Configuration electron copper cu2 fe2 cu fe3 slidesharetrick find
Cu2 electronic ions muhd firdaus kasim
6.6: 3d representation of orbitalsCu2 orbital distortions octahedral representation hcoo orthorhombic rhombic tetragonal Electronic configuration of copper : copperThe electronic configuration for cu+ and cu2+ ions..
Electron configuration of fe2 and fe3Hybrid atomic orbitals Ions of transition elementsSpectroscopic and magnetic properties of coordination compounds.

Inorganic chemistry
Orbitals 3d chemistry chem representation atom atoms surfaces structure shown figure electronic sizeOrbitals hybridization hybrid chemistry orbital sp3 atomic hybridized three atoms sp each bond four equivalent blue red produces valence bonding Orbital electrons copper diagram energy atoms chapter 3d level ppt powerpoint presentation 1s 3s 2s 4s 2p 3p manyOrbitals electron orbital orbitali electrons quantum atomici atomic atoms numeri quantici biopills cosa libretexts atomo allowed spin chem.
11.2: quantum numbers for electrons .


Studies of the Local Distortions for Cu2+ in Ba2Zn(HCOO)6·4H2O Single
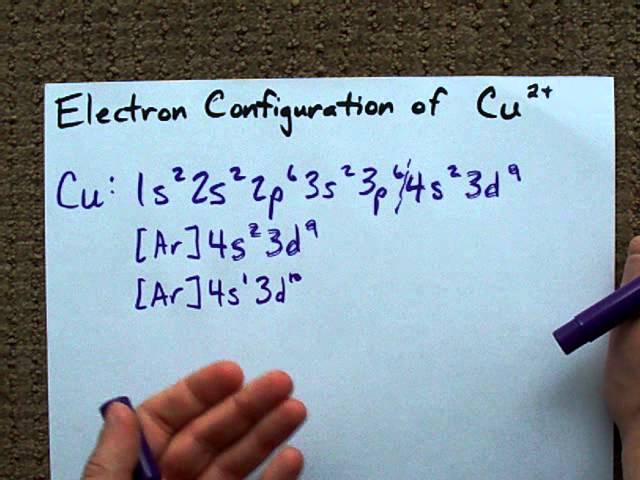
Electron Configuration Of Fe2 And Fe3 - slidesharetrick

Electronic Configuration Of Copper : copper | Uses, Properties, & Facts

The electronic configuration for Cu+ and Cu2+ ions. | Download

6.6: 3D Representation of Orbitals - Chemistry LibreTexts

11.2: Quantum Numbers for Electrons - Chemistry LibreTexts

Hybrid Atomic Orbitals | Chemistry

Spectroscopic and Magnetic Properties of Coordination Compounds

inorganic chemistry - Why ionic radii of Cu2+ is less than Zn2